Africa
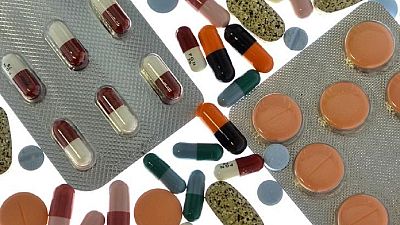
Medicines are an important part of the health care delivery system. Yet the incidence of fake drugs is a global headache which particularly affects developing countries.
According to the World Health Organization Africa Region, ‘’…fake medicines and medical products are sold at street corners, in open air markets or on unregulated websites in several countries in the African Region.’‘
‘‘These poor quality, unsafe medicines and products promote drug resistance and lead to loss of confidence in health professionals, pharmaceutical manufacturers and distributors and in health systems,’‘ they add.
Fighting fake drugs: WHO Road map
The WHO Regional Director for Africa, Dr Matshidiso Moeti, has proposed a strategy aimed at strengthening National Medicine Regulatory Authorities (NMRAs) in respective countries.
According to him, strong NMRAs will ensure that only safe, good quality and effective medical products are available in countries.
By 2018, countries are expected to ensure that a regular surveillance of all medical products circulating on the market is carried out.
During the same time frame, it is recommended that countries have access to certified quality control laboratories, and embark on joint reviews of applications for clinical trials.
The strategy further urges countries to establish governing bodies and quality management systems for NMRAs by 2025.
By the same period, application for clinical trials or marketing authorization of medical products should take a maximum of six months.
It is expected that countries will provide adequate human, financial and technical resources for the NMRAs to be functional and harmonize their regulatory practices with international recognized standards and initiatives.
Every two years, there will be an assessment of how countries are implementing the strategy based on a set of agreed indicators.
Nigeria’s senate wants life sentence for drug forgersWhere was the strategy discussed?
The strategy was presented at the annual meeting of Health Ministers from the WHO African Region.
During the 66th session of the WHO Regional Committee for Africa which is taking place in Addis Ababa from 19-23 August 2016.
Dr Matshidiso Moeti, WHO Regional Director for Africa speaks
“Functional regulatory systems ensure that medical products consistently meet international standards and are monitored from clinical evaluation to licensure and use.”
‘‘They play a critical role in protecting people’s health and strengthening health systems to contribute to Universal health Coverage.”












Go to video
Rwandan President Paul Kagame confirms bid to host Formula One race
Go to video
Mystery disease in Congo might be malaria - Authorities
01:16
António Guterres' vision for climate justice in Africa's G20 leadership
Go to video
Malaria cases surge in 2023, says WHO Report
01:01
Mystery disease spreads in DRC, mainly affecting children and the malnourished
01:12
Pope Francis Appoints 21 New Cardinals, Highlighting Global Inclusion