USA
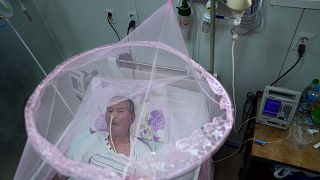
The United States Food and Drug Administration (FDA) has approved the first vaccine against chikungunya, developed by the European group Valneva, health authorities said.
The mosquito-borne disease, which causes fever and joint pains and can be fatal to newborns. Even although deaths are rare, it is seen as an “emerging global health threat”.
Approval by the FDA of the single-dose vaccine for people aged 18 and above is expected to speed up its rollout worldwide.
People in tropical and subtropical regions of Africa, Southeast Asia, and parts of the Americas are at the highest risk of infection.
Mosquitos carrying the chikungunya virus are endemic in these areas.
However, it has spread to new parts of the world, causing an increase in cases, including in Europe.
Valneva has also filed an application with the European Medicines Agency (EMA).
In the absence of any preventive treatment to date, the only way to protect oneself was to avoid being bitten.
According to the FDA, at least five million cases of chikungunya have been recorded in the last 15 years.










Go to video
UNICEF secures agreement to cut malaria vaccine costs
00:54
A third of Japan-donated Mpox vaccines wasted in DRC due to storage challenges
01:31
South Africa starts clinical trials on first locally developed oral cholera vaccine
01:07
WHO reports rapid spread of Mpox with 17 deaths in Africa over recent weeks
01:30
DRC: Health workers say Ebola outbreak in Kasai being brought under control
01:02
Cholera vaccination campaign launched in Darfur targeting over 1.8 million people